OU researchers help develop new tool in fight against rare genetic disease
Three Oakland University researchers are part of a team that developed a new method to screen FDA-approved drugs to determine if they could be repurposed or improved to help patients with Spinocerebellar ataxia type 5 (SCA5), a rare genetic disease of the nervous system.
SCA5 is caused by a mutation in the β-III-spectrin gene, which is critical for creating connections between neurons in the central nervous system. The mutation causes β-III-spectrin and a structural protein called actin to stick together, creating a traffic jam inside neurons and preventing them from carrying out their normal functions, such as controlling speech and movement.
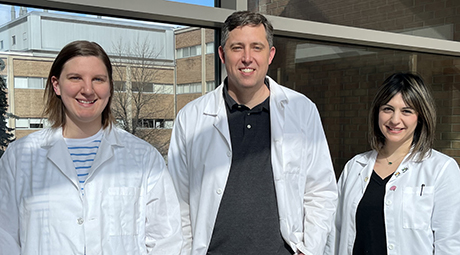
From left: Ph.D. student Alexandra Atang, Dr. Adam Avery, assistant professor of biochemistry, and Ph.D. student Sarah Denha helped develop a new method to evaluate drugs to see if they can be used to treat a rare neurological disorder called Spinocerebellar ataxia type 5.
The research team developed a new way to screen FDA-approved drugs against the mutated β-III-spectrin protein to find out if any can restore its normal function. The process uses cutting-edge spectroscopy to examine whether and how drugs change the interaction of mutant β-III-spectrin with actin. The drugs that dampen β-III-spectrin’s binding to actin initiate a unique florescent signal. The new method allows the team to evaluate 1,536 drugs in about six minutes, making it much more efficient than other drug screening processes.
Details of the new method were recently published in the Journal of Biological Chemistry. Dr. Adam Avery, an assistant professor in OU’s Department of Chemistry, oversaw the study, which is funded by the National Institutes of Health. Two doctoral students from Dr. Avery’s lab, Sarah Denha and Alexandra Atang, also worked on the study, along with researchers from the University of Minnesota.
“Our study is proof that the binding of this mutant spectrin to actin is a druggable target,” Dr. Avery said. “This work establishes that we can modulate this interaction using small molecule drugs.”
Of the 3,000 FDA-approved drugs analyzed thus far, two immediately stood out to the researchers for their high efficacy and potency: ginsenoside Rb1 and micafungin. However, this is just the beginning. The researchers plan to screen thousands more compounds in pursuit of an effective drug.
SCA5 is inherited and is sometimes called “Lincoln’s ataxia” because it has been passed down over 10 generations in one family descended from President Abraham Lincoln’s grandparents. Loss of coordination, impaired gait and slurred speech are just a few of the debilitating symptoms, which usually emerge between the ages of 20 and 30. There is currently no cure or even a targeted therapy for SCA5, which affects around 1,000 people in the U.S. but is a part of a group of diseases that plagues tens of thousands more.
The researchers plan to develop a cell culture and mouse model to more definitively test their hits from the drug screen. If the hits are successful in treating the disease in future models, they would be that much closer to a drug specific for SCA5 patients.
Though there is much work to be done, the team is optimistic about the prospects that this new development presents for studying mutations in β-III-spectrin-related proteins that cause other rare diseases like muscular dystrophy.
“Our findings may pave the way for the development of a new class of drugs that will provide relief to patients suffering from SCA5 and additional diseases due to mutations in spectrin-related proteins,” Dr. Avery explained. “Our technology is adaptable for drug discovery for treatment of other diseases associated with disrupted actin binding.”


 March 09, 2023
March 09, 2023